- Home
- Institut Néel
- Research teams
- Technical Groups & Services
- Work at the Institut
- Partnerships

My research is based on the synthesis of organosilica materials from original, home-made organosilanes for application in drug delivery, bioimaging, sensing or catalysis. An emerging thematic deals with the preparation and encapsulation of organic fluorophores in organosilica for bioimaging.
Organo(alkoxy)silanes are the main precursors for hybrid silica materials. Though many synthetic routes exist for these compounds, their sensitivity towards water is problematic when by-products or partial conversions are obtained and purification is needed. We introduced a very efficient method for the synthesis of such compounds based on the CuAAC reaction performed under strictly anhydrous conditions in aprotic solvents, using [CuBr(PPh3)3], a preformed copper(I) complex, which enables a very fast preparation of a wide range of organo(alkoxy)silanes.
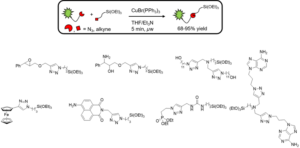
N. Moitra, J. J. E. Moreau, X. Cattoën and M. Wong Chi Man, Chem. Commun., 2010, 46, 8416–8418.
K. Bürglova, N. Moitra, J. Hodacova, X. Cattoën and M. Wong Chi Man, J. Org. Chem., 2011, 76, 7326–7333.
I am interested in the synthesis of silica nano-objects, such as mesoporous silica nanoparticles, periodic organosilica nanoparticles as well as core-shell organic@silicate nanoparticles.
In particular, I have worked on the controlled multiple functionalization of mesoporous silica. In collaboration with the SPCTS-IRCER laboratory in Limoges, we have shown that the CuAAC click reaction can be applied successively to alternate lines of alkyne- and azide-modified mesoporous silica microdots to anchor selectively two different fluorophores.
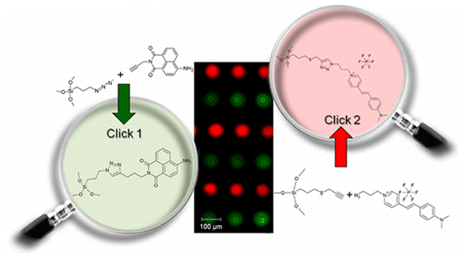
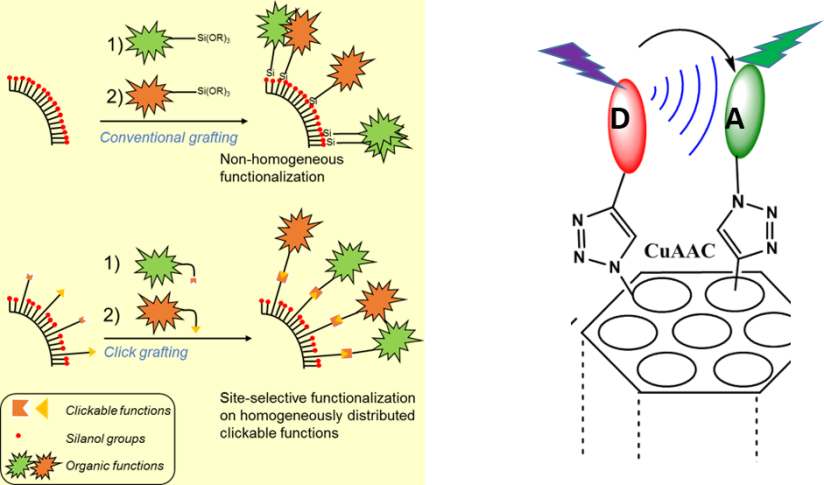
Mesoporous silica nanoparticles can also be selectively functionalized by two different organic groups able to communicate with each-other. The advantage of the click chemistry approach compared to the conventional grafting is to direct the functionalization towards sites that are randomly positioned on the surface.
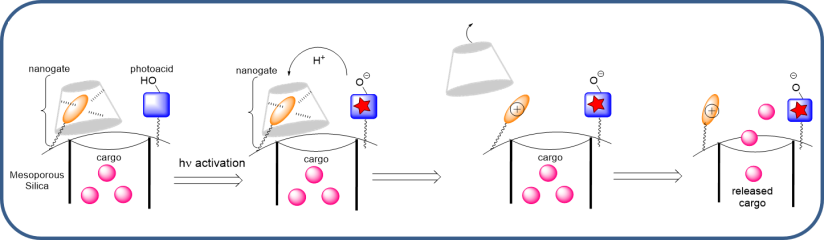
This concept allowed the formation of a nanomachine working through a proton transfer between a photoacid and a basic supramolecular nanovalve, in collaboration with JI Zink at UCLA.
In collaboration with JO Durand and M Wong Chi Man at Institut Charles Gerhardt in Montpellier, I am working on the development of periodic mesoporous organosilica nanoparticles (nanoPMOs) for nanomedicine.

NanoPMOs are hybrid mesoporous nanoparticles obtained by the sol-gel process from organo-bridged alkoxysilanes only, without any silica precursor. This confers them enhanced properties for drug delivery such as higher drug loading capacity.
Moreover, nanoPMOs can be made degradable thanks to the incorporation of breakable organic functions such as disulfide bonds in their structure.
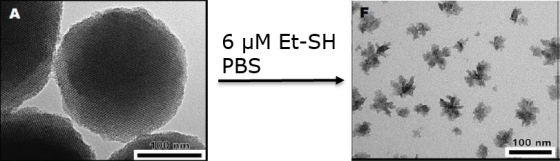
Silica-coated organic nanoparticles can be efficiently prepared using an original spray-drying approach developped in our laboratory:
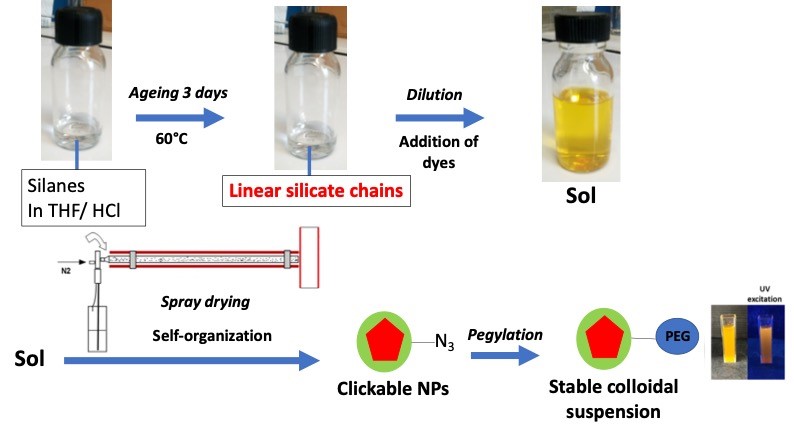
Process employed to obtain colloids of silica coated organic nanocrystals (red = organic nanocrystal; green = silica coating).
By spraying an aged sol containing a dissolved fluorophore, specifically deisgned to exhibit crystal-state emission in the red under two-photon excitation (Collab ENS Lyon), we can otain nanoparticles containing up to 40%wt of fluorophore, and exhibiting a core-shell morphology. The core is composed of an organic nanocrystal while the shell is a silica-based coating.
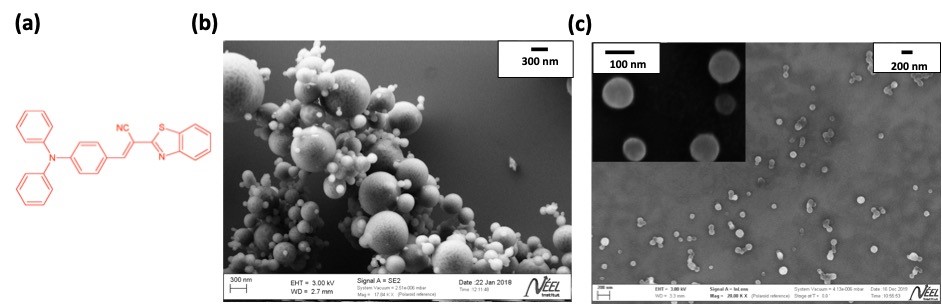
(a) Molecular fluorophore used in this study, engineered at ENS Lyon; (b) SEM images of silica-coated organic nanocrystals; (c) SEM image of the organic cores after silica etching.
These NPs can be functionalized in water to avoid dissolving the nanocrystal thanks to a CuAAC reaction on incorporated azide functions. This confers them the ability to be suspended in saline media incorporating important amounts of proteins.
These core-shell nanoparticles can be used for in vitro and in vivo bioimaging, with a strong brightness (Collab M Gary Bobo, IBMM Montpellier and O Pascual, INMG Lyon).
|
|
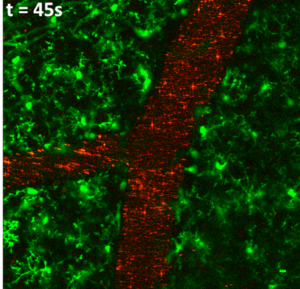 |
| In vitro imaging of MCF 7 cancer cells: cells were incubated with 10µg/mL of silica-coated organic nanoparticles under one-photon (left) and two-photon (right) excitation. Red: NPs; green: cell membranes. | In vivo Imaging of the brain vasculature of a mice expressing GFP in the microglia (green cells) using silica-coated organic nanoparticles (red) |
References:
S Shenoi-Perdoor et al, New Journal of Chemistry, 2018, 42, pp.15353-15360.
S Shenoi Perdoor et al, ACS Applied Nano Mater, 2020, 3, pp. 11933-11944.
We have recently developped a new setup for the nanocrystallization of organic coumpounds, which can be successfully employed to obtain colloids of organic nanocrystals with high crystallinity.
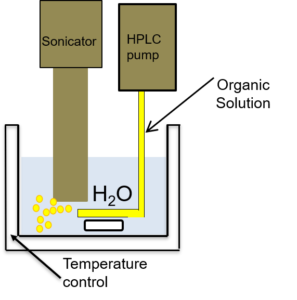
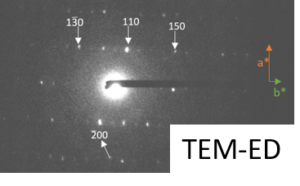
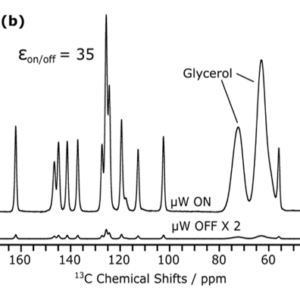
Setup developped for the nanocrystallization of organic compounds, and characterization of the crystallinity of sono-crystallized CMONS needles by Selected Area Electron Diffraction and 13C DNP-enhanced solid-state NMR (collab. G De Paëpe, CEA/MEM) [1].
This method can be applied to produce fluorescent colloids emitting in the red under two-photon excitation, which exhibit a very high brightness under two-photon excitation.
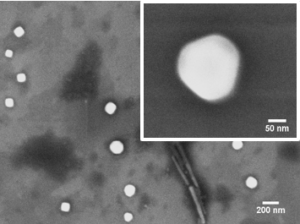
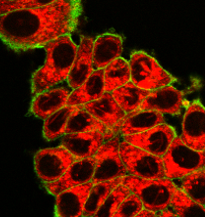
SEM micrograph of organic nanocrystals generated by sonocrystallization, and two-photon fluorescence image of MCF-7 cancer cells incubated with a 25 µg/mL colloid of a red-emitting dye irradiated at 900 nm (Collab. M Gary Bobo, IBMM Montpellier).
The nano-structuring of bridged silsesquioxanes was carried out back in 2001 by the formation of strong H-bonds between urea groups present in the framework of the organic bridging groups. In collaboration with JL Bantignies (Laboratoire Charles Coulomb Montpellier) and JR Bartlett (University of Sunshine Coast, AUS), we obtained nanostructured thin films of the same bridged silsesquioxanes using the spin-coating process. The films are composed of fibers in which the organic groups are well-aligned as shown by polarized infra-red measurements. The nanostructuring process occurs within fractions of a second as a result of the fast increase of concentrations in catalyst and silicate species due to the fast spinning.

Gaël de Paëpe, CEA-Grenoble/IRIG/MEM
Yoann Roupioz, CEA-Grenoble/IRIG/SyMMES
Cyril Picard, Liphy Grenoble
Farid Oukacine, Département de Pharmacochimie Moléculaire Grenoble
Michel Wong Chi Man, Philippe Trens and Jean-Olivier Durand, Institut Charles Gerhardt Montpellier
Magali Gary-Bobo, Institut des Biomolécules Max Mousseron, Montpellier
Jean-Louis Bantignies and Rozenn LeParc, Laboratoire Charles Coulomb Montpellier
Benoît Pichon and Sylvie Bégin-Colin, IPCMS Strasbourg
Yann Bretonnière, Akos Banyasz and Chantal Andraud, ENS Lyon
Martine Lejeune and Fabrice Rossignol, SPCTS Limoges
Luis D Carlos and Rute AS Ferreira, Universidade de Aveiro, PT
Veronica de Zea Bermudez, Universidade de Tràs os Montes e Alto Douro, PT
Roser Pleixats, Universitat Autonoma de Barcelona, ES
Jeffrey I Zink, University of California, Los Angeles, USA
John R Bartlett, University of Sunshine Coast, AUS
Sara Aldabe Bilmes, Universidad de Buenos Aires, ARG

Grenoble INP
La Prépa des INP : Chimie Organique 2ème année
Grenoble Alpes University
Master NanoChemistry, second year : Functional nanoparticles for medicine
Summer schools
2016 : Escuela NanoAndes Cali, Columbia: Lecture and practical works
2017 : 8va Escuela de Síntesis de Materiales : Proceso Sol-Gel Buenos Aires, Argentina: lectures and practical works
2019 : 9na Escuela de Síntesis de Materiales : Proceso Sol-Gel Buenos Aires, Argentina: lecture
2019 : Escuela NanoAndes Santiago, Chile: Lecture and practial works
51st International Chemistry Olympiad, Paris, July 19-29 2019 : Main writer of an exercise used for the final (TH5 : ‘Azobenzene –β-cyclodextrin complexes for the formation of nanomachines’).
Department: PLUM
Team: Optics and Materials (OPTIMA)
Status: Personnel Chercheur
Structure: CNRS
Position: Permanent
Email: xavier.cattoen@neel.cnrs.fr
Phone: 04 76 88 10 42
Office: F-211
